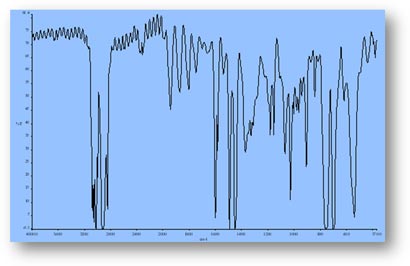
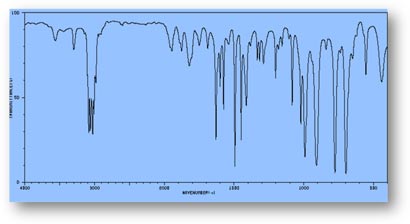
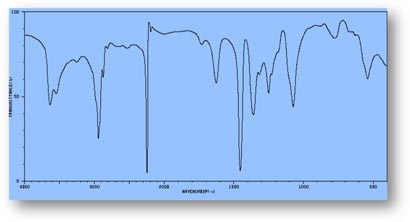
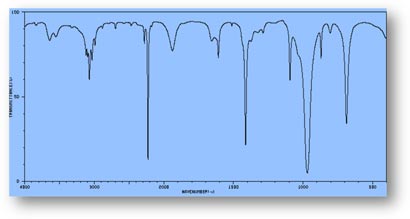
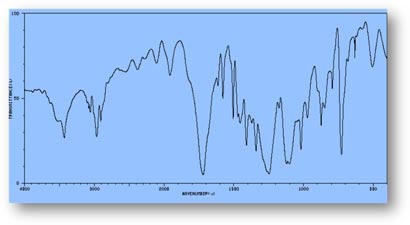
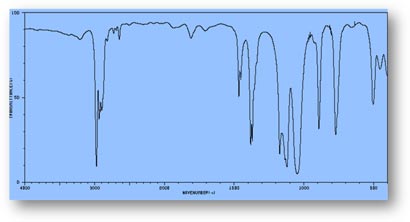
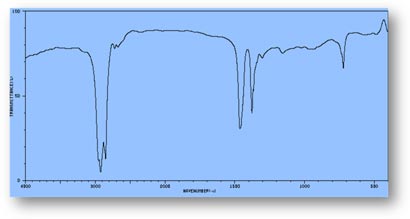
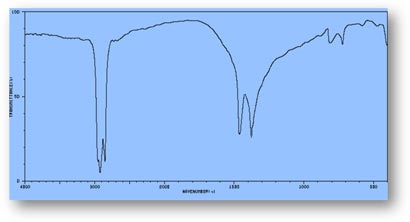
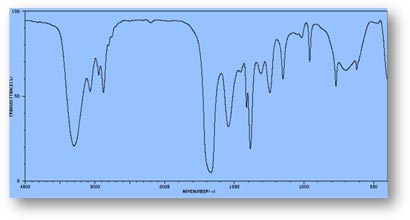
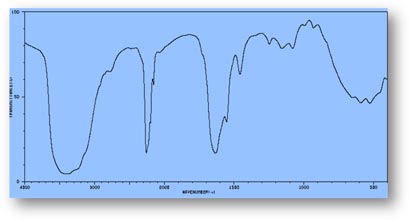
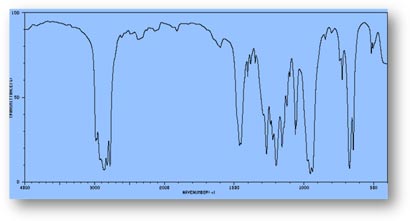
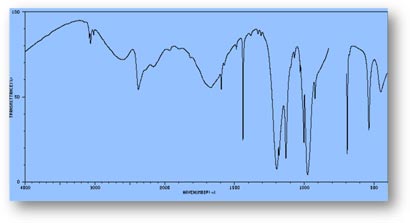
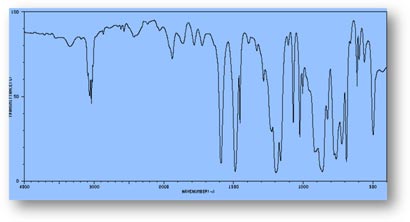
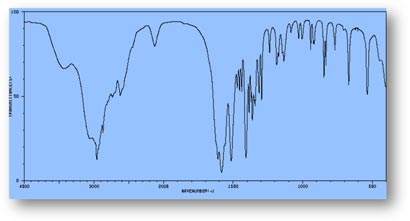
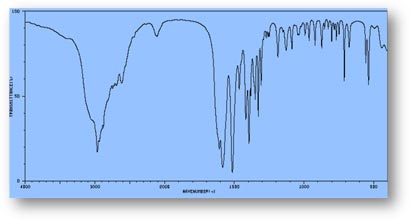
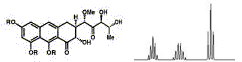FT-IR Spectra Samples
Silicone (Polydimethylsiloxane)
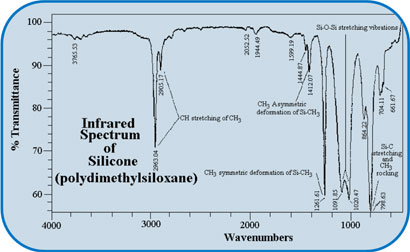
FT-IR (Fourier Transform Infrared) Spectroscopy is an Analytical Technique for many materials (solids, liquids or gas), pure or mixture of chemical compounds, pharmaceuticals etc. In this method of chemical analysis, one measures the infrared light absorption by the molecular vibrations in a material at different frequencies (expressed in wave number, cm-1) of infrared light. Based upon the frequency of infrared light or radiation absorbed, FT-IR can be categorized as:
- Far infrared (4 ~ 400 cm-¹) or Far-IR
- Mid infrared (400 ~ 4,000 cm-¹) or Mid-IR and
- Near infrared (4,000 ~ 14,000 cm-¹) or Near-IR
The mid-IR is commonly used as an extremely powerful analytical tool to detect the presence of molecular fragments or groups such as carbonyl C=O, amide NH2, etc. in the molecule or material.
List of Some FT-IR Spectra Examples:
- FT-IR of Polystyrene
- FT-IR of Styrene
- FT-IR of Polyacrylonitrile
- FT-IR of Acrylonitrile
- FT-IR of Polyethylene terephthalate
- FT-IR of Silicon Compound Tetraisopropyl Orthosilicate
- FT-IR of Boron Compound Boron Carbide
- FT-IR of Boron Compound Boron Nitride
- FT-IR of Nitrogen Compound N-methylformamide
- FT-IR of Nitrogen Compound Cyanamide
- FT-IR of Phosphorous Compound Hexamethylphosphorous triamide
- FT-IR of Phosphorous Compound Phenylphosphinic acid
- FT-IR of Phosphorous Compound Triphenyl phosphite
- FT-IR of Amino Acid L-Leucine
- FT-IR of Amino Acid L-Isoleucine_FTIR
List of Some FT-IR Spectra Examples:
| FT-IR of Polymer (Polyethylene glycol) | FT-IR of Polyethylene terephthalate | FT-IR of Polyvinyl alcohol |
| FT-IR of Silicon(II) oxide | FT-IR of Tetraisopropyl Orthosilicate | FT-IR of Acrylonitrile |
| FT-IR of PolyAcrylonitrile | FT-IR of Cyanamide | FT-IR of Boron Nitride |
List of Some FT-IR Less Common Samples:
| FT-IR of Hexamethylphosphorous triamide | FT-IR of Amino Acid L-Leucine | FT-IR of Boron Carbide |
| FT-IR of Styrene | FT-IR of Polystyrene | FT-IR of Boron Carbide |
Contact us for FT-IR Spectral Analysis and Consulting for a wide variety of materials at reasonable cost……
Infrared spectroscopy detects the vibration characteristics of chemical functional groups in a sample. When an infrared light interacts with the matter, chemical bonds will stretch, contract and bend. As a result, a chemical functional group tends to absorb infrared radiation close to a specific wave number range regardless of the structure of the rest of the molecule.
For example, the C=O stretch of a carbonyl group appears at around 1700 cm-1 in a variety of molecules. Hence, the correlation of this band wave number position with the carbonyl functional group in the chemical structure is used to identify the carbonyl functional group in a sample.
Infrared spectroscopy is extensively applied to various sample states such as liquid, gas, and solid-state matter to identify and to quantify unknown materials. It is a very good technique for identifying compounds and is used extensively to detect functional groups.
The FT-IR is routinely applied for the analysis of:
- Pure organic or inorganic compound analysis
- Impurity analysis
- Analysis of Polymers
- Analysis of biodiesels and gasoline and their additives
- Analysis of Cooking Oils
- Quantitative analysis of a compound in a mixture
- Analysis of thin films
An Example of Molecular Vibration is shown for the Benzene Molecule:
The Mid-FT-IR Spectrum of Benzene is shown below:
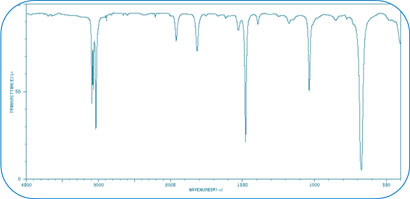
The IR spectrum for benzene, C6H6, has only four prominent bands because it is a very symmetric molecule. Every carbon has a single bond attached to a hydrogen atom. Each carbon is bonded to two other carbons and the carbon-carbon bonds are alike for all six carbons. The molecule is planar. The aromatic CH stretch appears at 3100-3000 cm-1 There are aromatic CC stretch bands (for the carbon-carbon bonds in the aromatic ring) at about 1500 cm-1. Two other bands are caused by bending motions involving carbon-hydrogen bonds. The bands for CH bends appear at approximately 1000 cm-1 for the in-plane bends and at about 675 cm-1 for the out-of-plane bend.
Contact us for FT-IR Spectral Analysis and Consulting for a wide variety of materials at reasonable cost……
FT-IR Spectra Examples:
Contact us for FT-IR Spectral Analysis and Consulting for a wide variety of materials at reasonable cost……
Characteristic FT-IR Band Positions of Functional Groups:
| Functional Group | Frequency Range (cm-¹) |
| OH stretching vibrations | |
| Free OH | 3610-3645 (sharp) |
| Intramolecular H bonds | 3450-3600 (sharp) |
| Intermolecular H Bonds | 3200-3550 (broad) |
| Chelate Compounds | 2500-3200 (very broad) |
| NH Stretching vibrations | |
| Free NH | 3300-3500 |
| H bonded NH | 3070-3350 |
| CH Stretching vibrations | |
| =-C-H | 3280-3340 |
| =C-H | 3000-3100 |
| C-CH3 | 2862-2882, 2652-2972 |
| O-CH3 | 2815-2832 |
| N-CH3 (aromatic) | 2810-2820 |
| N-CH3 (aliphatic) | 2780-2805 |
| CH2 | 2843-2863,2916-2936 |
| CH | 2880-2900 |
| SH Stretching Vibrations | |
| Free SH | 2550-2600 |
| C=-N Stretching Vibrations | |
| Nonconjugated | 2240-2260 |
| Conjugated | 2215-2240 |
| C=-C Stretching Vibrations | |
| C=-CH (terminal) | 2100-2140 |
| C-C=-C-C | 2190-2260 |
| C-C=-C-C=-CH | 2040-2200 |
| C=O Stretching Vibrations | |
| Nonconjugated | 1700-1900 |
| Conjugated | 1590-1750 |
| Amides | ~1650 |
| C=C Sretching Vibrations | |
| Nonconjugated | 1620-1680 |
| Conjugated | 1585-1625 |
| CH Bending Vibrations | |
| CH2 | 1405-1465 |
| CH3 | 1355-1395, 1430-1470 |
| C-O-C Vibrations in Esters | |
| Formates | ~1175 |
| Acetates | ~1240, 1010-1040 |
| Benzoates | ~1275 |
| C-OH Stretching Vibrations | |
| Secondary Cyclic Alcohols | 990-1060 |
| CH out-of-plane bending vibrations in substituted ethylenic systems | |
| -CH=CH2 | 905-915, 985-995 |
| -CH=CH-(cis) | 650-750 |
| -CH=CH-(trans) | 960-970 |
| -C=CH2 | 885-895 |
Contact us for FT-IR Spectral Analysis and Consulting for a wide variety of materials at a reasonable cost